Anode and Cathode Sign, Symbol, Example, Polarity, Difference
The terms anode and cathode come when we talk about electrodes. But do you know what is an electrode? The electrode is a substance or conductor which makes contact between an electric circuit and a nonmetallic part such as an electrolyte in an electrolytic cell. In both types of cells such as galvanic cells and electrolytic cells, electrodes are used. The anode and cathode are opposite to each other.
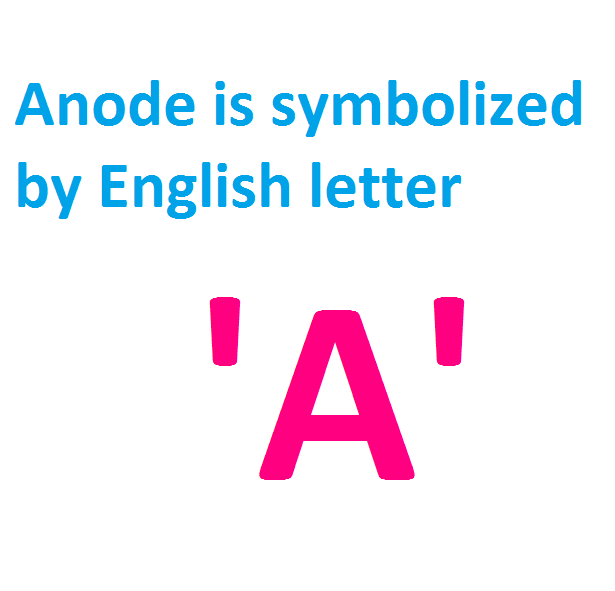
Anode Symbol and Sign
The anode is symbolized by the English letter 'A'
Cathode Symbol and Sign
The anode is symbolized by the English Letter 'K'
Example of Anode
In a PN junction diode, the positive terminal where the electric current enters is known as the anode terminal. A Platinum Electrode can be an example of anode. When a battery is discharging its anode acts as a negative terminal as current flows through it inwards. But when the battery is charging its anode acts as a positive terminal as it receives current from the external charging circuit. In fact, the term Anode mostly heard while studying about the construction of a battery or a cell.
Examples of Cathode
In a PN Junction diode, the negative terminal from where electric current flows out is known as a cathode. Graphite Electrode can be an example of cathode. When a battery is discharging the cathode acts as a positive terminal. And when the battery is charging the cathode act as a negative terminal. So, a Cathode does not have a particular polarity, it depends upon the device, application area, or system.
Difference Between Cathode and Anode
1. A cathode is an electrode in an electrolytic or galvanic cell through which electricity flows out from the electrolyte to the external circuit. On the other hand, Anode is an electrode in an electrolytic or galvanic cell through which electricity moves into the electrolyte from the external circuit.
2. The cathode is the negative-sided electrode whereas the anode is the positive-sided electrode.
3. In an electrolytic cell a reduction reaction happens in the cathode while in that electrolytic cell, an oxidation reaction happens in the anode. So you may understand cathode handles reduction reaction whereas anode handles oxidation reaction.
4. In a galvanic cell the cathode acts as an anode. On the other hand, in a galvanic cell, the anode acts as a cathode.
5. Cathode mostly acts as an electron acceptor whereas anode mostly acts as an electron donor.
6. Cathode is mainly indicated by the English letter 'K' whereas Anode is indicated by the English Letter 'A'.
7. In an electrolytic cell anode is positive but in a galvanic cell, the anode is negative. On the other hand, in an electrolytic cell cathode is negative but in a galvanic cell, the cathode is positive.
Now, you may think about how to identify the anode and cathode in a circuit or cell. It is very simple. For example, we can talk about a battery that is a basically galvanic cell. Also, we know that an anode is an electrode through which electrons go to the external circuit and a cathode is an electrode through which electrons go into the cell. As the current flows from the positive terminal to the negative terminal that means electrons flow from the negative terminal to the positive terminal. So here the positive terminal or positive electrode will be the anode and the negative terminal or negative electrode will be the cathode.
Read Also: